2025-08-21 ヒューストン大学(UH)
<関連情報>
- https://uh.edu/news-events/stories/2025/august/08212025-carbon-capture.php
- https://www.nature.com/articles/s41467-025-61525-3
膜なし電気化学的に仲介されたアミン再生による二酸化炭素捕集 A Membraneless Electrochemically Mediated Amine Regeneration for Carbon Capture
Ahmad Hassan,Mohsen Afshari & Mim Rahimi
Nature Communications Published:09 July 2025
DOI:https://doi.org/10.1038/s41467-025-61525-3
Abstract
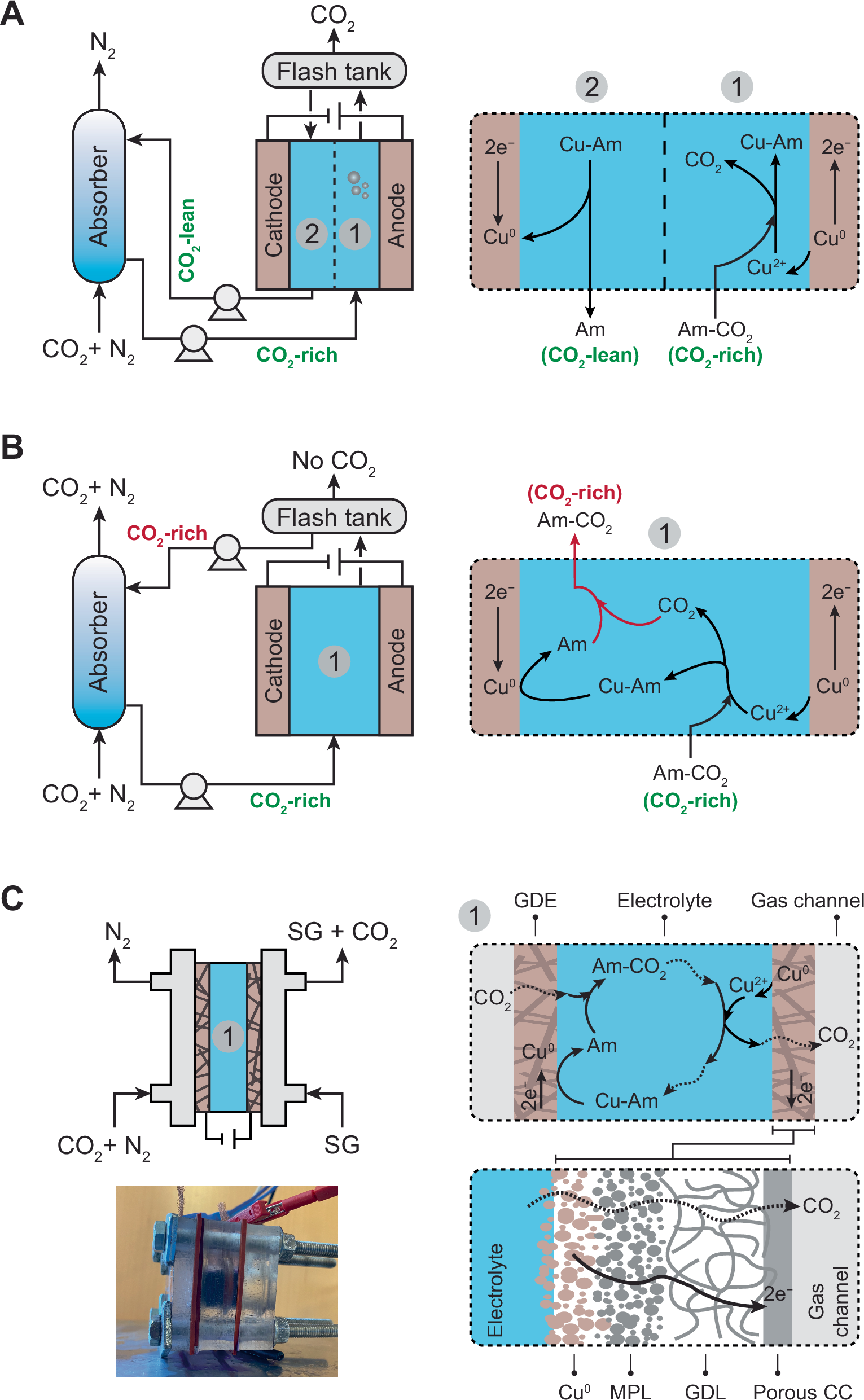
Electrochemical carbon capture (ECC) processes offer efficient, scalable, and modular alternatives to conventional thermal-based methods. Among ECCs, electrochemically mediated amine regeneration (EMAR) reached higher technology readiness levels, moving from small-scale laboratory studies toward pilot-scale implementations. Previous EMAR systems rely on ion-selective membranes, which contribute significantly to the cost and present challenges for long-term operation. This study presents a membraneless EMAR system by fundamentally redesigning the process configuration and using gas diffusion electrodes (GDEs) as both the anode and cathode. This setup eliminates the membrane and the need for additional equipment such as the absorption column, flash tank, and pumps, significantly reducing the process footprint and simplifying the flow diagram. Two GDE configurations, mesh-attached and electrodeposited, are tested and compared in terms of CO2 removal efficiency, current density, and energy consumption. Electrodeposited GDEs achieve CO2 removal efficiencies above 90% with energy consumption as low as 60 kJ/mol CO2. A techno-economic analysis estimates a levelized cost of capture of ~$70/tonneCO2, compared to $137/tonneCO2 for conventional EMAR. Further improvements in current density and removal efficiency may enable costs below $50/tonneCO2. These results position the membraneless EMAR as a potentially promising approach for cost-effective and scalable point-source carbon capture.
二酸化炭素捕集とエネルギー貯蔵のためのバナジウム還元フロープロセス A Vanadium Redox Flow Process for Carbon Capture and Energy Storage
Mohsen Afshari,Abdelrahman Refaie,Prince Aleta,Ahmad Hassan,and Mim Rahimi
ACS ES&T Engineering Published: January 29, 2025
DOI:https://doi.org/10.1021/acsestengg.4c00631
Abstract

Climate change mitigation by decreasing worldwide CO2 emissions is an urgent and demanding challenge that requires innovative technical solutions. This work, inspired by vanadium redox flow batteries (VRFB), introduces an integrated electrochemical process for carbon capture and energy storage. It utilizes established vanadium and ferricyanide redox couples for pH modulation for CO2 desorption and absorbent regeneration. The developed process consumes electricity during the daytime─when renewable electricity is available─to desorb CO2 and charge the cell, and it can regenerate the absorbent for further CO2 absorption while releasing electricity to the grid during nighttime when solar power is unavailable. This research explores the process fundamentals and scalability potential, through an extensive study of the system’s thermodynamics, transport phenomena, kinetics, and bench-scale operations. Cyclic voltammetry (CV) was utilized to study the thermodynamics of the process, mapping the redox profiles to identify ideal potential windows for operation. The CV results indicated that an overpotential of approximately 0.3 V was required for driving redox reactions. Additionally, polarization studies were conducted to select the practical operating potential, identifying 0.5 V as optimal for the CO2 desorption cycle to provide sufficient polarity to overcome activation barriers in addition to the Nernstian potential. Mass transfer analysis balanced conductivity and desorption efficiency, with a 1:1 ratio identified as optimal for redox-active species and background electrolyte concentration. To further enhance the kinetics of the redox reactions, plasma treatment of electrode surfaces was implemented, resulting in a 43% decrease in charge transfer resistance, as measured by electrochemical impedance spectroscopy (EIS) analysis. Finally, a bench-scale operation of the system demonstrated an energy consumption of 54 kJ/mol CO2, which is competitive with other electrochemical carbon capture technologies. Besides its energy competitiveness, the process offers multiple additional advantages, including the elimination of precious metal electrodes, oxygen insensitivity in flue gas, scalability inspired by VRFB technology, and the unique ability to function as a battery during the absorbent regeneration process, enabling efficient day-night operation.